We use cookies to give you a better experience on our website. Learn more about how we use cookies and how you can select your preferences.
CRC for Cell Therapy Manufacturing – feel-good work
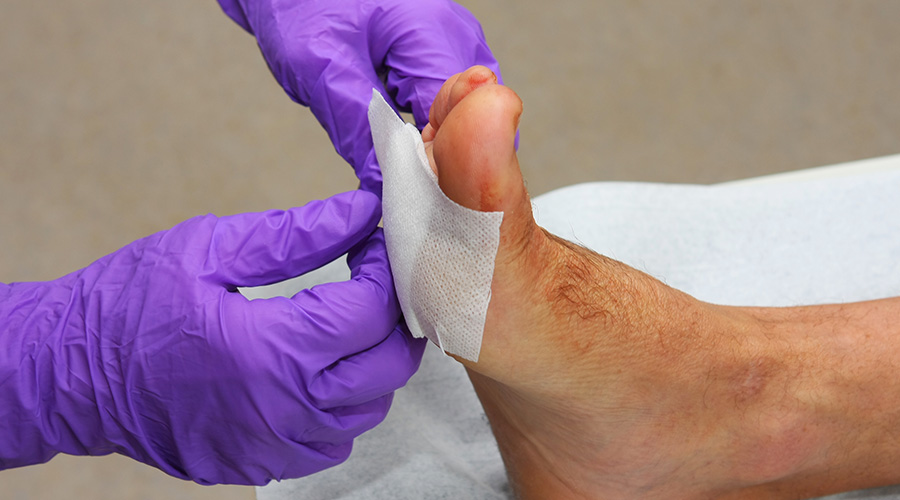
CTM CRC’s work is exciting for the field of regenerative medicine and cell therapy
Stem cell treatment for chronic wounds
The Australian Government’s Cell Therapy Manufacturing Cooperative Research Centre (CTM CRC) has created a dressing to deliver adult stem cells to wounds and will, in the first instance, be used for the treatment of chronic foot ulcers in diabetics.
CTM CRC determined that the delivery of stem cells directly to the wound site was a key to producing an economical treatment for chronic ulcers and wounds. The brilliance of this targeted delivery approach is the use of a unique coating to the surface of the dressing, so that it can grow and detach therapeutic adult stem cells, and the dressing can then be applied directly to the ulcer or wound.
Normally, a cell therapy approach would necessitate the injection of a large number of stem cells around a fragile and painful wound. CTM CRC’s dressing presents a kinder alternative to injections and is also more efficient since far fewer cells are required through this targeted approach. This means that the number of cells required for effective treatment can be reduced by hundreds of thousands, if not millions, resulting in significant cost savings.
The dressing developed by CTM CRC biomaterial scientists and cell biologists has been tested in a number of wound models – including small and large animals, with encouraging results. The adult stem cell being used with CTM CRC’s patented dressing has been developed by one of CTM CRC’s industry partners in the United States and is currently in clinical trials for a number of medical conditions.
Diabetic foot and leg ulcers are a major problem in Australia and around the world and if untreated, will often lead to amputation. An efficient and cost effective cell therapy for these ulcers will mean more patients can be treated. It is a very exciting development for this type of condition.
There are more than 200 million difficult-to-treat chronic wounds in individuals suffering from diabetic foot ulcers, pressure ulcers and chronic venous leg ulcers. In Australia, there are more than 400,000 chronic wounds at any given time.
The dressing is in the final phases of pre-clinical testing, which if successful, will lead to a first-in-man clinical trial in Australia in 2017.
CTM CRC’s scientists and researchers are also working on the development of a 3-D scaffold for the safe and consistent production of a sufficient number of immune (T) cells for use in the treatment of a range of immune conditions.
Immunotherapies are one of the fastest growing areas of cell therapy and are being developed for the treatment of a range of conditions, including certain cancers. Cell-based immunotherapies require a patient’s immune cells to be collected and grown in numbers large enough for a therapeutic dose before being administered back to the patient.
CTM CRC’s novel technology aims to improve this process by providing a unique surface that can be integrated with existing commercial systems that are used to produce large numbers of immune cells. The technology, before being applied to human clinical use, is being tested in a number of commercial systems, through collaborations with public and private organisations in the United Kingdom and United States.
The testing phase of the immunotherapy cell therapies project involves collaborations with commercial and public sector organisations in Australia, the United States and the United Kingdom.
Download the CTM CRC customer story
pdf · 0.20 MB